Trigonelline (TRG)
Introduction
Trigonelline (1-methylpyridin-1-ium-3-carboxylate, C7H7NO2, TRG) was first isolated from the seeds of fenugreek (Trigonella foenum-graecum L.).(1) It is a solid, crystallizable pyridine alkaloid, with a molecular weight of 137.14 g/mol and an octanol-water partition coefficient of -2.53.(1) It is a small, highly hydrophilic, and thermolabile phytochemical naturally occurring in numerous plants as a secondary metabolite.(1)
TRG, as the most abundant alkaloid, is found with the highest content in coffee beans, which makes coffee the most important commercial source of TRG.(1,2) TRG contents in coffee vary from 0.2 to 63 g/kg depending on many factors, including country of origin, coffee species and cultivar, part of the coffee plant, age of the plant part, applied industrial production processes, used preparation methods, water content of the sample, pH condition of the sample media, and analytical method used.(1) TRG content in green coffee beans ranges 2.3-34.2 g TRG/kg green coffee beans, and 3.1-7.5 g/kg in roasted coffee.(1)
Health Effect
TRG shows various beneficial roles in many pathological conditions, including modulation of glucose and lipid homeostasis, recovery from neurological impairments such as neurodegenerative disorders, ischemia-induced brain damage, depression, cognitive decline, and diabetic peripheral neuropathy, mitigation of DM and its complications, protection of cardiovascular system, liver, lungs, kidney, gastric system, and skin, and inhibition of tumor cell proliferation and migration.(3)
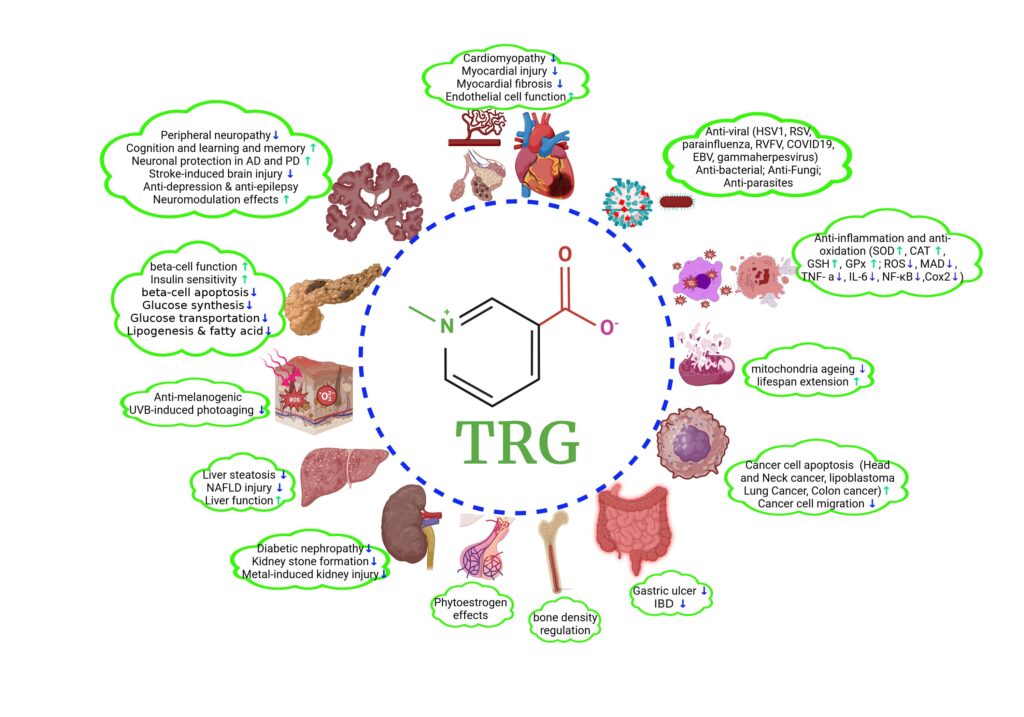
Mechanism
TRG can potentially bind to PPARγ, NGF, and several neurotransmitter receptors, restoring glucose and lipid homeostasis, promoting neuronal survival, and modulating neuronal activity. TRG can act as a phytoestrogen to stimulate ER. TRG can potentially bind to GSK, MPO, and Aβ, executing inhibitory effects. TRG can interfere Nrf2 nuclear translocation, prevent EMT, and suppress tyrosinase activity.(3)
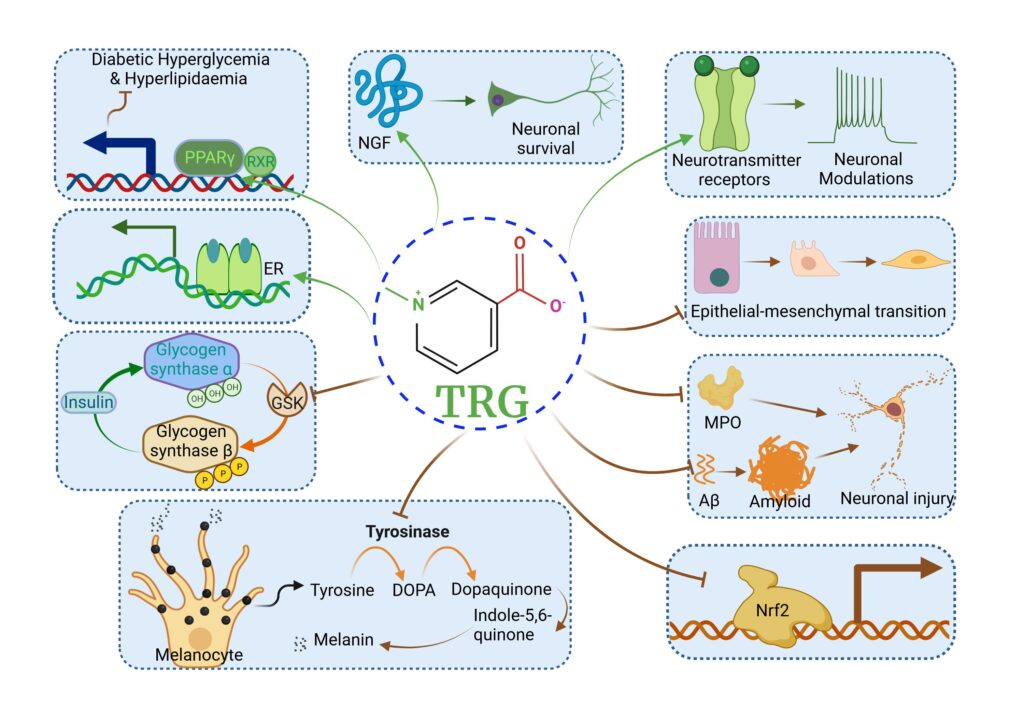
Safety
TRG ingested as a component of coffee appears to be safe for human health, based on the safe traditional use of these products.(1) No evidence of adverse effects following acute exposure to TRG was identified.(1) In addition, the lack of abortifacient activity or teratogenicity in animals confirms the safety of TRG during pregnancy.(4)
Reference
- Konstantinidis N, Franke H, Schwarz S, Lachenmeier DW. Risk Assessment of Trigonelline in Coffee and Coffee By-Products. Molecules. 2023 Apr 14;28(8):3460. doi: 10.3390/molecules28083460. PMID: 37110693; PMCID: PMC10146819.
- Taguchi H, Sakaguchi M, Shimabayashi Y. Trigonelline Content in Coffee Beans and the Thermal Conversion of Trigonelline into Nicotinic Acid during the Roasting of Coffee Beans, Agricultural and Biological Chemistry, 1985, 49:12, 3467-3471, DOI: 10.1080/00021369.1985.10867295
- Nguyen V, Taine EG, Meng D, Cui T, Tan W. Pharmacological Activities, Therapeutic Effects, and Mechanistic Actions of Trigonelline. International Journal of Molecular Sciences. 2024; 25(6):3385. https://doi.org/10.3390/ijms25063385.
- Kandhare AD, Thakurdesai PA, Wangikar P, Bodhankar SL. A systematic literature review of fenugreek seed toxicity by using ToxRTool: evidence from preclinical and clinical studies. Heliyon. 2019 Apr 24;5(4):e01536. doi: 10.1016/j.heliyon.2019.e01536. PMID: 31049444; PMCID: PMC6482331.
